The Unseen Shield: How Flame Retardant Masterbatches Keep Us Safe
2025-12-05
Imagine a world where everything, from the wiring in your walls to the plastic casing of your TV, caught fire easily. Sounds terrifying, right? Thankfully, a quiet hero works behind the scenes to prevent this: the Flame Retardant Masterbatch.
What Exactly is a Masterbatch?
Before we dive into fire safety, let’s break down the term Masterbatch. It’s not a complicated ingredient; it’s a concentrated mixture of pigments (colors) or additives (like flame retardants) encapsulated in a plastic polymer.
Why Use a Masterbatch Instead of Powder?
Masterbatches are the preferred way to add these ingredients to bulk plastic during manufacturing for several key reasons:
- Better Dispersion: The additive is already mixed into a plastic carrier, ensuring it spreads evenly throughout the final product. No clumps or weak spots!
- Cleaner Handling: Manufacturers deal with dust-free plastic pellets instead of fine, potentially messy or harmful powders.
- Accurate Dosing: It’s easy to measure and add the exact right amount, ensuring consistent product quality and performance.
- Efficiency: The high concentration means a smaller amount of masterbatch is needed compared to pure additive, saving space and cost.
The Science of Flame Retardancy
A flame retardant is an additive designed to inhibit or suppress the process of combustion. When a plastic product is exposed to heat or an open flame, the flame retardant masterbatch kicks in, using one or more chemical mechanisms to stop the fire.
How Flame Retardants Fight Fire
Flame retardants work by interfering with the “fire triangle,” which consists of three necessary elements: heat, fuel (the plastic itself), and oxygen.
- Cooling the Fuel: Some retardants release non-combustible gases (like water vapor) when heated. This process absorbs heat, effectively cooling the material down below its ignition temperature.
- Creating a Protective Layer (Charring): Certain retardants, often phosphorus-based, react in the heat to form a thick, foamy, or solid carbon layer called “char.” This char acts as an insulating barrier, blocking heat from reaching the underlying, unburned plastic and cutting off the fuel supply.
- Interrupting the Flame Chemistry: In the fiery gaseous phase, combustion occurs via free radical reactions. Halogenated (containing chlorine or bromine) flame retardants release active chemical species that scavenge these high-energy free radicals, disrupting the chain reaction that sustains the flame. This mechanism essentially “puts out” the flame in the gas above the material.
Where These Masterbatches Are Used
Flame retardant masterbatches are vital components in modern manufacturing, silently protecting us in countless applications. They are essential wherever fire safety standards are strict.
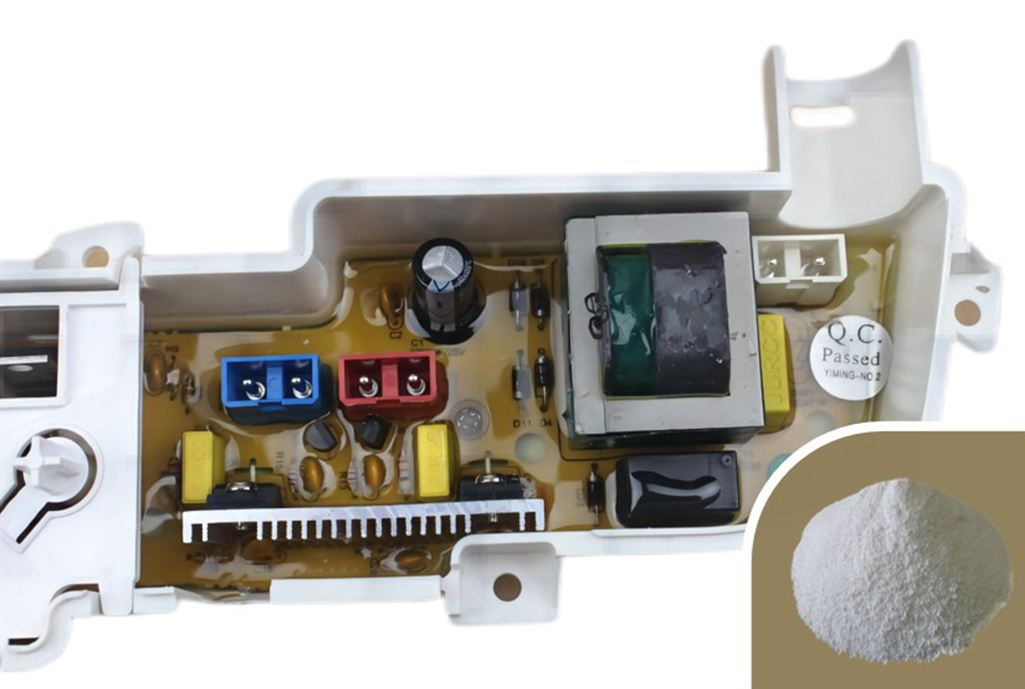
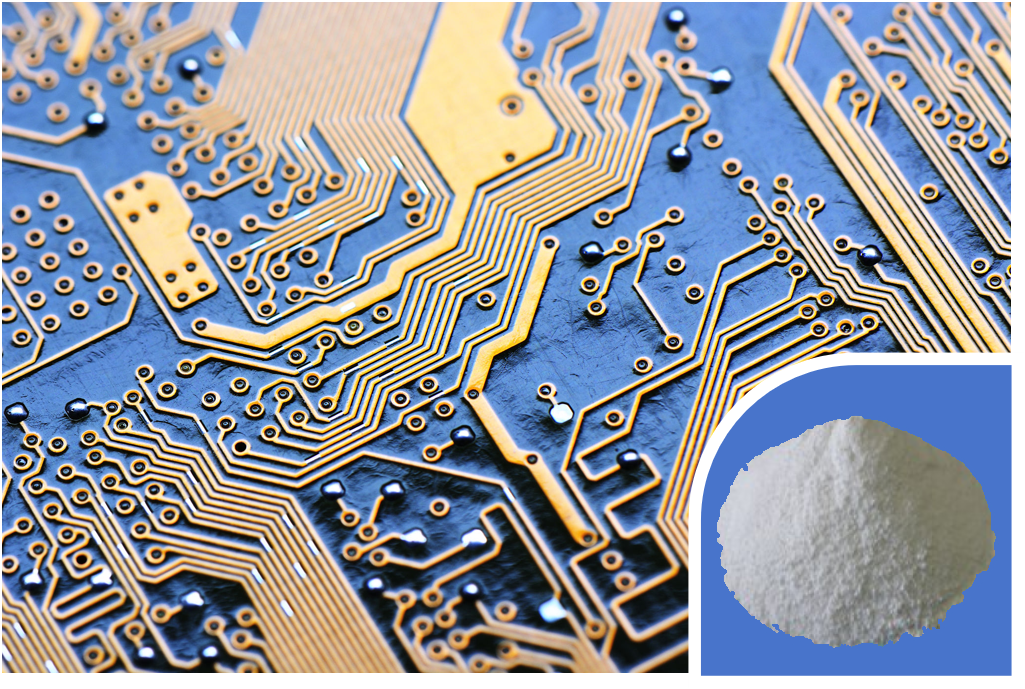
- Electronics and Appliances: Used in television casings, computer monitors, circuit boards, and connectors to prevent fires that could be sparked by electrical shorts.
- Construction: Incorporated into materials like cable insulation, pipes, flooring, and roof membranes to slow the spread of fire within buildings.
- Transportation: Used in the interior components of cars, trains, and aircraft (seats, dashboards, wiring) to enhance passenger safety.
- Textiles and Furniture: While often used in coatings, flame retardant additives are sometimes incorporated into plastic fibers or foam components to meet strict flammability regulations.
Thanks to the integration of flame retardant masterbatches, the everyday plastic items we rely on are far less likely to become sources of ignition, giving us precious extra minutes to escape in the event of a fire. They are a clear example of how advanced chemistry creates a safer world.